News & Events
what's going on in the world of neurosurgery
Neurosign Leans into Future of Consumables with New Strategy
Neurosign®, the IONM brand of Technomed Europe, announced recently a new strategy to emphasize the design and production of the next generation of nerve monitoring instruments, including stimulating probes, electrodes, and stimulating surgical instruments used around the world for intraoperative nerve monitoring.
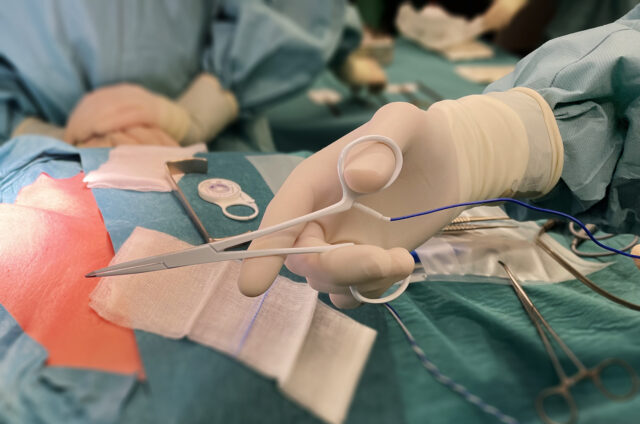
Neurosign’s innovative stimulating dissectors give surgeons the capabilities of a monopolar probe in surgical dissectors.
Known for the quality of its instruments, Neurosign®’s announcement begins to wind down more than three decades of producing both nerve monitors and the accessories associated with those monitors.
“When we entered the intraoperative nerve monitoring market more than 30 years ago, our goal was to serve surgeons with quality tools to aid them in the protection of patient nerves,” said Ronnie Stolec-Campo, CEO of Technomed Europe. “This strategic shift allows us to continue that mission by focusing on our strengths and placing the best instruments into the hands of surgeons no matter which monitor they are using.”
More than 30 years ago with its previous parent company, Magstim® Company Ltd., Neurosign® pioneered the world of intraoperative nerve monitoring with the N100 dual-channel nerve monitor. Years later, that evolved into the V4 Intraoperative Nerve Monitor which debuted in the UK in the mid-2010s.
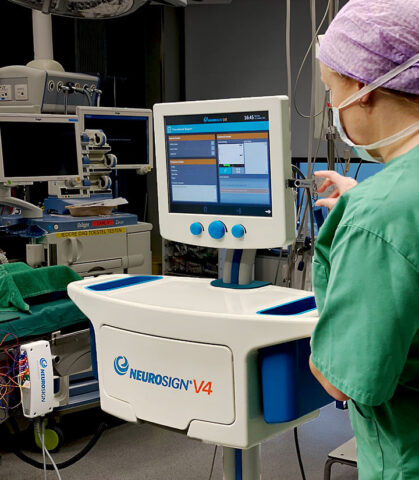
After more than three decades of producing an intraoperative nerve monitor, Neurosign will shift its focus exclusively to the design and production of consumable nerve monitoring instruments.
While not an easy decision to bring such a notable chapter in the company’s history to a close, Stolec-Campo said Neurosign now has a greater opportunity to redirect resources to the development and distribution of next generation cost-effective and reliable consumable nerve monitoring instruments.
“Our marketing and development teams will direct their attention to the evolution of intraoperative nerve monitoring instruments, working with surgeons and their teams to develop accessories that help protect the nerve integrity and function for their patients while satisfying healthcare systems’ needs for value-based products,” she said.
Sales of the V4 Intraoperative Nerve Monitor will continue but gradually wind down.
“We are grateful for the trust that surgeons have placed in us over the decades to preserve what matters to patients,” Stolec-Campo said. “We look forward to many more years of collaboration for the benefit of patients and those caring for them.”




