News & Events
what's going on in the world of neurosurgery
FDA Clears Neurosign Intraoperative Nerve Monitor To Help Surgeons Preserve Nerves
Used in thousands of surgeries globally, the V4 4- and 8-channel now in the US
Minneapolis, MN – (May 24, 2021) – Neurosign, one of the leading intraoperative nerve monitoring solutions used by surgeons worldwide, has officially launched the V4 System in the US. For 30 years, Neurosign has been used globally in thousands of ENT procedures including mastoid, thyroid, parathyroid and parotid surgeries. Neurosign worked with leading surgeons to create the V4, used primarily for cranial nerves, skull base surgeries and spinal nerve roots.
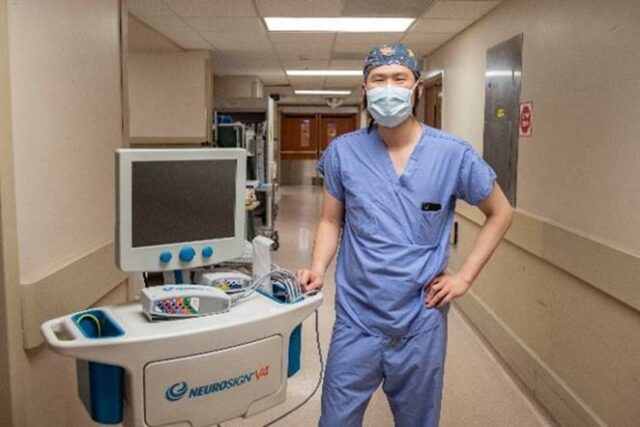
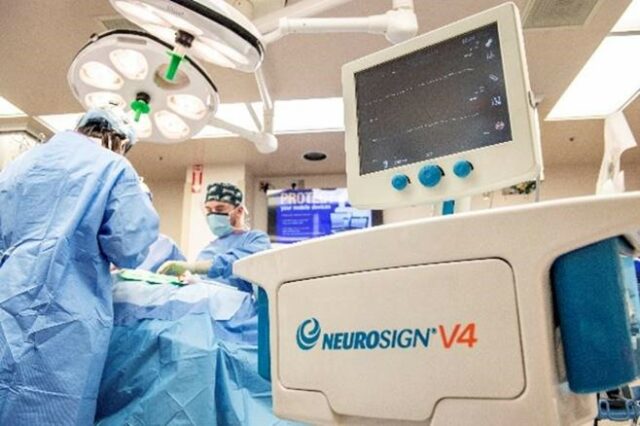
WVU Photo/Brian Persinger
On March 17, 2021, Neurosign gained FDA clearance for the V4 4-channel and 8-channel nerve monitoring system and it is already being used on patients for nerve identification and preservation in the USA. Dr. Jeffson Chung, a surgeon and Assistant Professor of Otolaryngology, Director of QI and Safety, West Virginia University Hospital, recently used the V4 in successful thyroidectomy and facial nerve surgeries. For nerve identification and stimulation, Dr. Chung used the Neurosign Bipolar probe.
Lothar Krinke, PhD, CEO Welcony, Inc., said, “We have many years of experience listening to surgeons and developing systems that solve challenges. The V4 is the next iteration of advanced nerve monitoring that focuses on patient safety and preserving the patient’s critical nerve structures. We are proud to bring the Neurosign V4 system to the US market.”
Dr. Chung added, “I use neuromonitoring as an early warning signal to help with the identification of the laryngeal nerve. It is an additional tool I use to help minimize risk.”
Neurosign technologies have been used in hospitals and clinics in the USA and worldwide. According to Dr. Krinke, this system provides USA surgeons with an intuitive, precise, reliable, and cost-effective technology that still provides a full range of new technology solutions to preserve nerves.
Both surgeons and patients look to Interoperative Nerve Monitors to improve outcomes. According to a study by Bergenfelz, “IONM reduced the risk of permanent vocal cord palsy. No bilateral recurrent laryngeal nerve injury occurred following IONM.” *
The Neurosign® V4 Intraoperative Nerve Monitor is a multi-channel touch screen nerve monitor is intended for locating and identifying cranial and peripheral motor and mixed motor-sensory nerves during surgery, including spinal nerve roots.
Indications for 8 channel Neurosign V4 Intraoperative Nerve Monitor include, Intracranial surgeries, Extracranial surgeries (e.g. parotidectomy), Intratemporal surgeries (e.g. mastoidectomy), Extratemporal surgeries, Neck surgeries (e.g. thyroidectomy), Spinal surgeries (e.g. tethered cord, degenerative treatments, pedicle screw procedures, cauda equina, fusion cages, rhizotomy, and open/percutaneous lumbar procedures), Surgeries to the upper and lower extremities (e.g. tethered cord, pedicle screw procedures, cauda equina).
The V4 allows for substantial personalization of its functions which can be saved in personalized surgeon profiles with customized procedure settings. The nerve monitor has self-checking routines to ensure that the unit is working reliably. Key features include Intuitive workflow, (push 3 icons and the device is ready for monitoring), clear digital EMG signal to quickly analyze critical information, create patient reports with captured information, default configuration for quick start mode, safe pre-set current values, excellent artefact rejection and Neurosign disposables which provide a significant saving against other devices.
The Neurosign V4 is available for delivery through Neurosign’s USA based sales and education team. To learn more about the Neurosign V4, visit www.neurosign.com or call 844 200 4473. See fact sheet for technical specifications. The Neurosign V4 launch is part of a series of new innovations coming to market in 2021 through Welcony, Inc.
About Welcony
Globally, Welcony technologies have supported thousands of research labs, clinics, hospitals and universities that focus on mental health, brain disorders, cognitive neuroscience and neuromonitoring. Key brands include Magstim Magnetic Stimulation, MagstimEGI high-density EEG, Technomed Clinical Neurophysiology and Neurosign Intraoperative Nerve Safety Monitoring.
REFERENCE
*Bergenfelz A, Salem AF, Jacobsson H, Nordenström E, Almquist M; Steering Committee for the Scandinavian Quality Register for Thyroid, Parathyroid and Adrenal Surgery (SQRTPA). Risk of recurrent laryngeal nerve palsy in patients undergoing thyroidectomy with and without intraoperative nerve monitoring. Br J Surg. 2016 Dec;103(13):1828-1838. doi: 10.1002/bjs.10276. Epub 2016 Aug 18. PMID: 27538052.
Media Contact: Mark Sejvar, mark.sejvar@magstimegi.com, 323-363-3530




